Conectores de pequeno diâmetro ISO 80369 para líquidos e gases em aplicações de saúde
Requisitos de ensaios mecânicos para conectores luer conforme ISO 80369
Escrito por: Landon goldfarb
Atualizado: 14 de novembro de 2025
A ISO 80369 é uma norma internacional que especifica os requisitos de ensaio para conectores de pequeno diâmetro utilizados em dispositivos médicos. Seu objetivo principal é prevenir conexões incorretas e garantir a segurança do paciente. A norma especifica ensaios mecânicos que verificam a segurança e confiabilidade do conector sob condições clínicas.
Estes ensaios incluem:
- Força de tração axial
- Resistência a vazamentos
- Fissuração por tensão
- Verificação dimensional
Para fabricantes e laboratórios de ensaios contratados, compreender estes protocolos é essencial para conformidade e integridade do produto em todos os tipos de conectores, incluindo
- Enteral
- Respiratório
- Inflação de manguito de membro
- Neural
- Intravascular ou hipodérmico
Visão geral dos conectores luer
Os conectores luer são conectores de pequeno diâmetro que são universalmente utilizados em aplicações médicas para garantir compatibilidade entre componentes como seringas e cateteres. Eles permitem conexões confiáveis independentemente do fabricante, suportando funções críticas como administração de medicamentos e retirada de fluidos corporais.
Falhas nestes conectores podem levar a complicações perigosas, incluindo:
- Contaminação de medicamentos
- Incapacidade de fazer uma conexão segura entre dispositivos
Devido ao seu uso generalizado, a ISO 80369 é uma das normas mais frequentemente testadas na indústria de dispositivos médicos.
Tipos de conectores Luer
Os conectores luer podem ser macho ou fêmea e vêm em dois tipos:
- Luer Slips: Tampas mantidas no lugar pelas forças de atrito introduzidas por um encaixe cônico de 6°
- Luer Locks: Tampas com conexão rosqueada, oferecendo uma fixação mais segura
O tipo de conector luer afeta:
- Requisitos de ensaio
- Resultados esperados
- Especificações de interface para conectores de referência
Os conectores de referência são especificados na ISO 80369 com dimensões precisas e tolerâncias para as conexões de acoplamento ao dispositivo sob ensaio.
Testes de acordo com a ISO 80369
Partes da norma
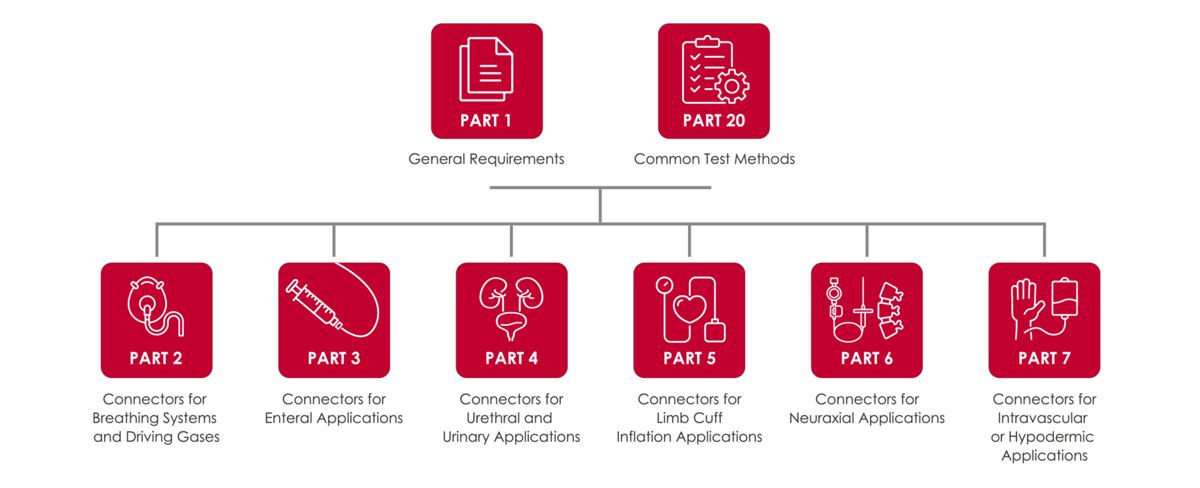
A ISO 80369 inclui oito partes, cada uma abordando aplicações específicas e métodos de ensaio:
- Parte 1: Requisitos gerais
- Parte 2: Conectores para sistemas de respiração e gases de condução
- Parte 3: Conectores para aplicações enterais
- Parte 4: Conectores para aplicações uretrais e urinárias
- Parte 5: Conectores para aplicações de inflação do manguito do membro
- Parte 6: Conectores para aplicações neuraxiais
- Parte 7: Conectores para aplicações intravasculares ou hipodérmicas
- Parte 20: Métodos de teste comuns
A Parte 1 delineia requisitos gerais e contexto para conectores luer e suas aplicações.
As Partes 2 a 7 incluem detalhes específicos da aplicação, como geometria do conector e critérios de ensaio.
A Parte 20 inclui os procedimentos completos para cada ensaio definido pela norma. Também fornece métodos alternativos para obter dados variáveis – em oposição a dados de atributo – reduzindo efetivamente o tamanho da amostra necessária.
Esta modificação altera os resultados do ensaio de uma avaliação qualitativa – se vazamento ou dano foi visível – para um valor quantitativo. Esta mudança pode exigir que o dispositivo seja testado até a falha, requerendo forças e torques mais altos.
Na prática, o número de dispositivos de ensaio pode ser limitado – como é frequentemente o caso para verificação de projeto de produto. Usar dados variáveis pode ser útil nestas circunstâncias.
Anexos de ensaio principais
Nove anexos de ensaio definem como avaliar a qualidade do fechamento. Os ensaios simulam casos de uso típicos e extraordinários usando forças aplicadas, torques e pressões internas.
- Anexo B: Vazamento por queda de pressão
- Anexo C: Vazamento de líquido sob pressão positiva por queda de gota
- Anexo D: Vazamento de ar sob pressão subatmosférica
- Anexo E: Quebra de tensão
- Anexo F: Resistência à separação da carga axial
- Anexo G: Resistência à separação por desparafusamento
- Anexo H: Resistência à anulação
- Anexo I: Desconexão por desparafusamento
- Anexo K: Vazamento de ar durante aspiração (novo na versão 2024 da norma)
Desafios nos ensaios ISO 80369
- Múltiplas Configurações de Ensaio - Métodos manuais reduzem o rendimento e requerem mudanças frequentes de equipamento. Integrar o sensor de pressão e gerador na máquina de ensaio universal otimiza o fluxo de trabalho e economiza espaço no laboratório.
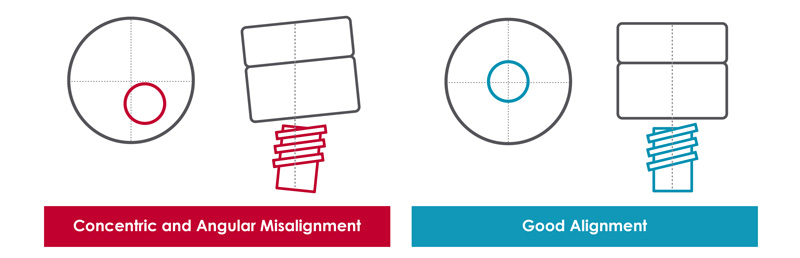
- Alinhamento do Sistema - Alcançar as forças de montagem e torques necessários depende de alinhamento concêntrico e angular preciso. Desalinhamento pode levar a resultados não conformes. Usar uma mesa XY para reduzir desalinhamento ajuda a garantir dados confiáveis.
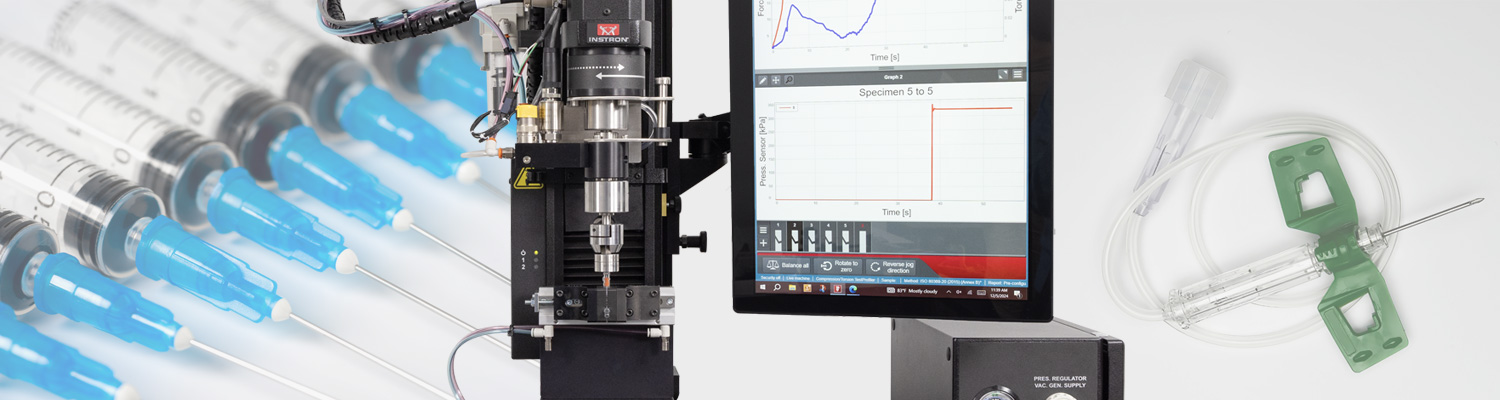
Sistema de ensaio de materiais recomendado
Para conformidade com ISO 80369, recomendamos o sistema de ensaio universal Instron 68SC-1 com o Torsion Add-On 3.0. Esta configuração permite ensaios biaxiais – combinando forças axiais e torcionais – para avaliar o desempenho mecânico de conectores luer.
As características do sistema 68SC-1 incluem:
- Configurações para ensaios de pressão positiva e negativa
- Dispositivos intercambiáveis para seringas e conectores e hubs personalizados
- Design modular para ensaio de uma ampla gama de dispositivos
O sistema de ensaio ISO 80369 da Instron suporta os Anexos B a I, fornecendo a flexibilidade necessária para suportar ensaios de seringas, hubs IV, conectores e mais.

Configuração de ensaio para ISO 80369
- Conector de referência
- Dispositivos específicos do dispositivo
- Mesa XY manual
- Célula de carga biaxial
- Complemento de Torção
- Controlador de pressão
Resumo
A ISO 80369 garante a segurança e confiabilidade de conectores de pequeno diâmetro na área da saúde. A conformidade requer compreensão dos tipos de conectores, métodos de ensaio e capacidades do equipamento. Ao otimizar fluxos de trabalho e usar sistemas de ensaio avançados, os laboratórios podem melhorar a eficiência e precisão.
Para garantir conformidade, sempre leia a norma ISO 80369 completa.
Visite nosso guia para ensaios de seringas, cartuchos e frascos para saber mais.



