BLUEHILL® CENTRAL
Simplify Your Testing Management
Bluehill Central software is a laboratory management tool that enables centralized, remote management of Bluehill Universal software applications associated with multiple Instron test frames. The software allows you to remotely manage all Bluehill Universal users, test templates, results, file revision approvals, and audit trail data from multiple Instron systems.
Implementations Since Launching
Connected with Bluehill Central
Take a short quiz to find out.
Bluehill Central Architecture
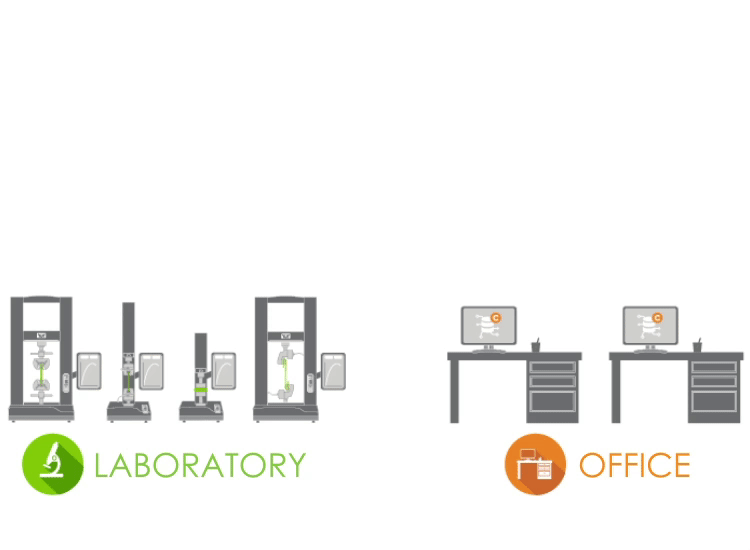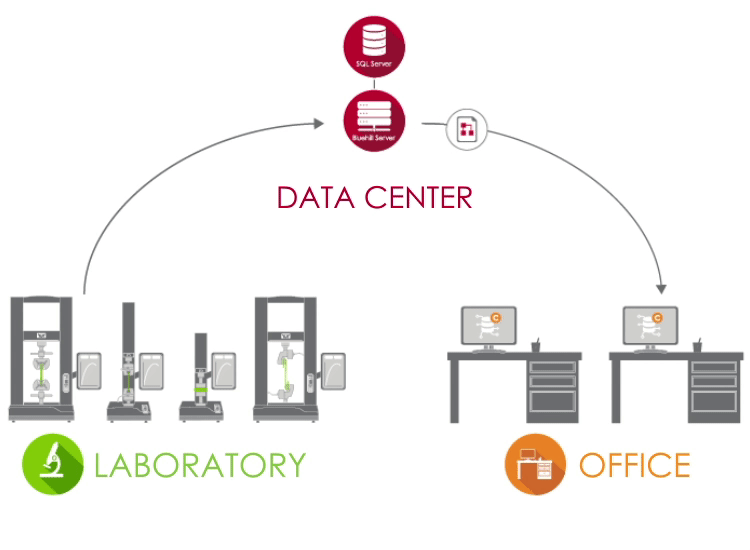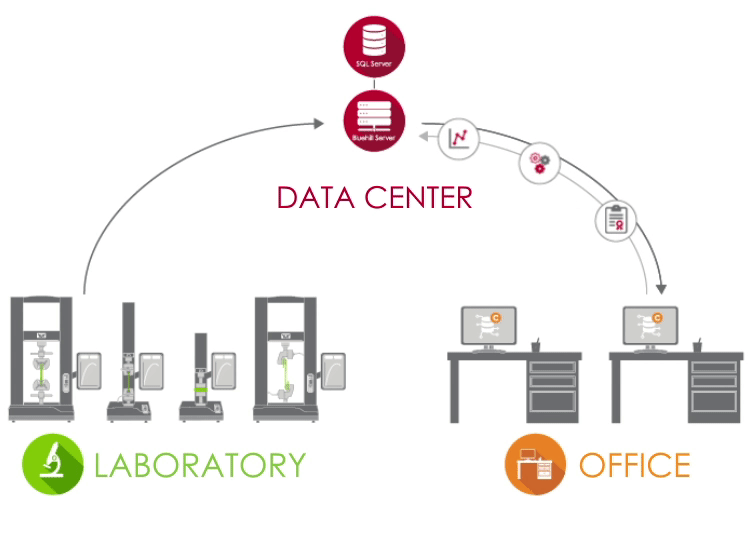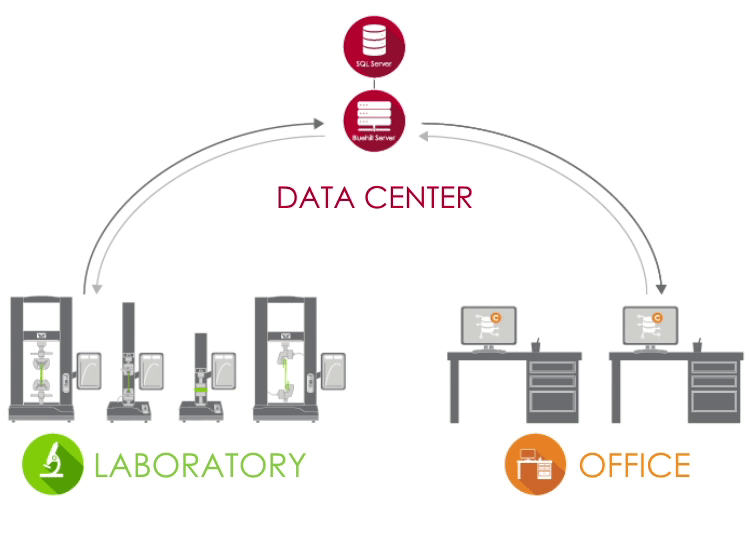
Bluehill Central uses a client / server network to store all shared data, files, and settings on a Microsoft® SQL Server database. When Bluehill Universal is connected to your lab’s Bluehill Central server, each testing system sends and receives data from the centralized database, eliminating the risk of variation and the burden of locally managing each system.
 |
LABORATORY In the lab, PCs that are running Bluehill Universal and are connected to your corporate network can access the Bluehill Central database through the Central plug-in. |
 |
DATA CENTER A data center managed by your IT group can host the SQL Server database that centrally stores the Instron data and the Bluehill Server application that handles all transactions between clients and the database. All clients and servers are connected via your corporate network. |
 |
OFFICE Outside of the laboratory, you can remotely access files and settings from the Bluehill Central viewer. You're also able to use offline Bluehill Universal licenses to edit test methods and view sample data. |
Learn more in our Guide for Lab Managers and IT Admins.
Bluehill Central allows groups of users to be organized into teams that share common files and settings. An operator’s permissions and access to files are defined by their team. This gives your lab the flexibility to support different workflows and settings depending on the group using the system.
MEMBERS | Individual users with personalized permissions
TESTING SYSTEMS | Instron frames in your lab connected to Bluehill Central
FILES | Bluehill methods, report templates, PDF reports, or other Instron related files
AUDIT TRAIL | A record of actions performed by team members
SETTINGS | Security type, file approvals, and other team-level settings
Learn about security options in Bluehill Central.
Which Modules Are Right for Your Lab?
The Lab Management module contains Bluehill Central’s file repository, which is stored on the database server. Connected Instron frames can access the database to run controlled test methods or export results.
![]()
Each Bluehill file contains its history, detailing all changes associated with each revision of the file.
![]()
Storing and retrieving files from the database is simple with upload and download functions.
![]()
Limit which users can make changes or access files by configuring read and write access for files and folders.
![]()
Operators can continue testing if connection to the database is lost by making critical files or folders available offline in Bluehill Universal.
The Traceability module in Bluehill Central allows you to remotely review and electronically sign off on test method modifications, sample data, and other changes to Bluehill files that occur on Instron systems. File approvals and many other user actions in Bluehill Central and Bluehill Universal are automatically captured in a centralized audit trail to verify who, did what, when and why on any connected Instron system. Note: The Traceability module displays data from Instron systems with the Traceability + Lab Management (Central plug-in), an add-on
to Bluehill Universal.
![]()
Perform file reviews from all connected test systems from the comfort of your desk.
![]()
Query, filter, and print the audit trail of all connected test systems.
![]()
The detail pane provides further information associated with the selected audit trail entry.

See how Traceability in Bluehill Central compares to standard Security in Bluehill Universal and local Traceability.
Learn how Traceability within Bluehill Central can help you meet the technical requirements of FDA 21 CFR Part 11.
The TrendTracker module in Bluehill Central accelerates the data analysis workflow of your lab. An intuitive interface allows you to quickly search, display, and analyze results over time and across multiple samples and test systems. Say goodbye to tedious file searching and copy-pasting of data.
Multi-Sample Data Analysis
Perform data analysis on results from multiple samples produced by multiple test systems.
Customizable Queries
Create queries defined by test parameters or results that provide insights to trends and outliers.
Visually Plotting
Plot a variety of chart types to visualize data sets.
Export Data
Export the data to a CSV file and open it in an application, like Minitab®, for further analysis.
Learn more about the statistics and charts available in TrendTracker.
Discover how TrendTracker empowers you to make faster, data-driven decisions.
Take a quick tour of TrendTracker and see how easy it is to set up, monitor trends, and uncover insights.