How to Navigate the Transformation of USP 381 and Its Replacements
The United States Pharmacopeia (USP) provides standards and guidance related to the development and production of medicine. The USP is more commonly referenced within formulation organizations, but it does provide defined methodologies for evaluating primary and secondary containers for drug delivery. More specifically, we are looking at USP <381> and its replacements: <382>, <1381>, and <1382>.
USP Chapter <381>, which discussed the requirements and tests associated with elastomeric components in contact with drug products, will be replaced on December 1, 2025. Pharmaceutical and drug delivery device organizations will have to comply with the updated tests in the newly developed chapters before the older tests are formally obsoleted.
- The design of the packaging/delivery system as it relates to the individual component
- The interaction between the primary containers and the intended pharmaceutical dosage
- The anticipated environmental conditions the product is subject to throughout the complete product life cycle
- The administration setting and expected patient interaction with the device

Additionally, this holistic view of the product provides flexibility to modify testing protocols for functional performance as necessary to better align with the considerations discussed above, given sufficient justification.
USP <382> provides more detailed information regarding the test methodology and relevant products, while USP <1382> offers a more thorough examination of the differences between evaluating an individual component and a component as part of a complete delivery system. Additionally, it provides supplementary information regarding testing, including ISO references, sample sizes, procedures, data analysis, and reporting.
USP <1381> is primarily focused on an evaluation of the component materials, looking at physiochemical, biocompatibility, and extractable properties. As such, it is not particularly relevant from a mechanical testing perspective. However, it is important to note that the decisions made in material and coating selection will have an impact on functional device performance.
Across all standards are references to elastomeric components that are present in the following device types.

Stoppers
Syringes
Needle shields
Plungers
Tip caps
Cartridges
Plungers
Seals
Blow Fill Seal (BFS) Containers
Seals
Infusion Products
Access ports
When applicable, the penetration device will be another variable that impacts the test procedure. For elastomeric components that are pierced to access the drug product, the device that performs the action will typically be characterized as either a needle or a spike access device. The product’s use case and method of drug preparation will often dictate which is utilized. For example, many elastomeric seals are intended to be accessed multiple times via needle, as in the case of cartridges in insulin pens. Generally, spike access is single use intended for transferring drug from one container to another, preparing a dose, or accessing an infusion bag.
Penetration Force
Purpose: To evaluate the force required to pierce the elastomeric component simulating real-world usage.
Critical Results: Maximum Penetration Force [Variable]
Key Points:
- Consider the potential impact that liquid could have on the results and justify the inclusion or exclusion of liquid from the container under test.
- Use a new needle for each test.
- If the expected piercing device designation is not available, use the recommendation provided within the USP.
- Fixturing should be designed to ensure the piercing device is perpendicular to and concentric with the center of the seal.
- Testing is performed at 200 mm/min across all device types.
| USP Test Method | Device Type | ISO Reference |
|---|---|---|
| Penetration Force | Vial | ISO 8362-2 ISO 8362-5 ISO 8871-5 |
| Bottle | ISO 8536-2 ISO 8536-6 |
|
| BFS Container | ISO 15747 | |
| Infusion Bag | ISO 15759 | |
| Spike Retention | Vial | N/A |
| Bottle | ISO 8536-2 ISO 8536-6 |
|
| BFS Container | N/A | |
| Infusion Bag | ISO 15747 | |
| Plunger Break Loose & Glide Force | Syringes | ISO 11040-4 ISO 11040-8 ISO 7886-1 |
| Cartridges | ISO 11608-3 ISO 13926-2 |
|
| Plunger Seal Integrity | Syringes | ISO 11040-4 ISO 11040-8 ISO 7886-1 |
| Cartridges | ISO 11040-2 ISO 11608-3 ISO 13926-2 |
|
| Tip Cap & Needle Shield Functional Suitability | Syringes | ISO 11040-4 ISO 11040-8 |
Spike Retention and Sealing Capacity
Purpose: To evaluate the performance of the spike access device in a simulation of expected use including insertion, maintained connection, and removal.
Critical Results: Confirmation of Connection [Attribute], Spike Removal Force [Variable], Visual Confirmation of No Leakage [Attribute]
Key Points:
- USP offers alternate procedures that do not require the use of a mechanical test system and opt for hanging weights.
- For vials and bottles, the containers should be filled to 50% of nominal capacity during testing.
- Spike insertion is defined as a manual process within the guidance.
- Fixturing should be designed to ensure the piercing device is perpendicular to and concentric with the center of the seal.
- Testing on infusion bags offers additional challenges, as applying a set internal pressure is required between parallel plates. It is difficult to directly correlate applied system load and internal pressure of a flexible container. Supplementary benchmarking with a pressure sensor may be required to determine a defined relationship between applied load and internal pressure.
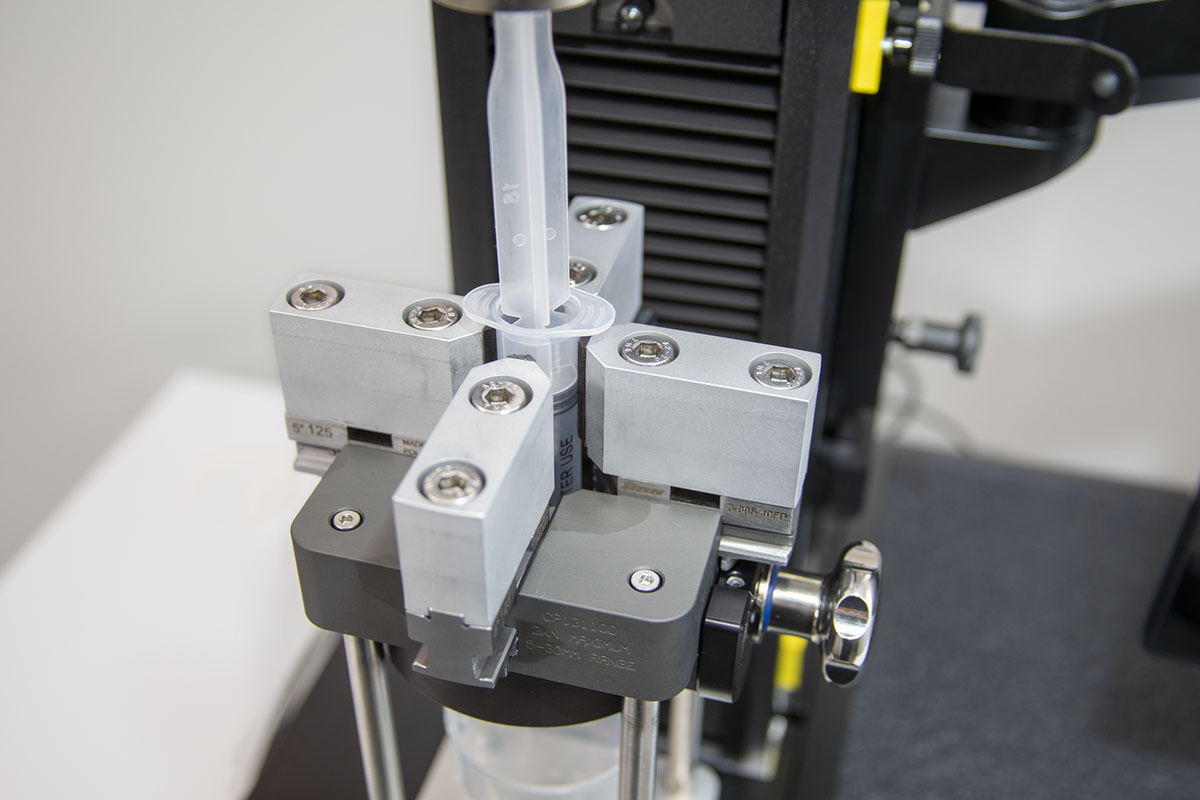
Plunger Break Loose and Glide Force
Purpose: To evaluate the performance of the device plunger, simulate the forces required to initiate movement, and then maintain that motion throughout the barrel. These forces can be indicative of dimensional tolerances of elastomeric components, siliconization of the barrel, drug viscosity, and other variables. When pre-filled syringes are utilized in needle-based injection systems like autoinjectors, these forces will help specify the required forces for the device activation mechanisms.
Critical Results: Break Loose Force [Variable], Glide Force [Variable], Observation of Stick-Slip Behavior [Attribute]
Key Points:
- USP provides fewer quantitative test parameters due to the number of variables that can contribute to device performance. The intent is to lean more heavily on ISO standards for method guidance.
- USP specifically requires comment on the presence of stick-slip behavior, which can indicate issues with the device such as out-of-specification component dimensions or non-uniform barrel lubrication.
- Dual chamber syringes require evaluation of each individual chamber.
- Fixturing should be designed to ensure the probe is well aligned with the plunger to avoid off-centered or angled loading.
- It is important to report both maximum and minimum glide force to indicate the consistency of the barrel lubrication.
- For cartridge-based devices, fixturing should allow break loose and glide force to be measured when used in conjunction with a double-sided needle.
Plunger Seal Integrity
Purpose: To evaluate the seal performance of the elastomeric closure when under expected loading conditions.
Critical Results: Visual Confirmation of No Leakage [Attribute]
Key Points:
- USP provides four procedures, each specific to a particular device, with slight differences in loading pressure and time.
- For pre-filled staked needle syringes, care must be taken to seal the tip of the needle.
- For syringes, the visible check is only required for leakage past the plunger, whereas for cartridges, leakage could occur at the plunger or the seal of the container.
- Internal container areas are utilized to convert applied load into an internal pressure for the test system.
- Adjusting system load cell gains might be necessary to tune the system response when in a force control mode.
- For non-dental cartridges, an applied loading equation is specified as opposed to a target pressure.
Target Force = p×d2 p = 0.64 N/mm2 d = nominal inner diameter [mm]

Tip Cap and Needle Shield Functional Suitability Tests
Purpose: To evaluate the performance of the various closure systems of pre-filled syringes. The test methods will depend on whether the syringe has a staked needle or a luer connector for injection. Tip caps are used for devices with luer connectors, and rigid needle shields (RNS) are used for staked needle designs.
Critical Results: Rigid Needle Shield Pull-Off Force [Variable], Tip Cap Unscrewing Torque [Variable]
Key Points:
- The USP specifies that the fixture for RNS pull-off avoids any damage or deformation of the cap, more closely aligning with method 2 of ISO 11040-4.
- Evaluating tip caps will require systems with torsion capabilities.
- Fixturing should be designed to ensure the upper fixture is well aligned with the closure to avoid off-centered or angled loading.
Learn more about drug delivery and container testing.
Explore systems and accessories for testing drug delivery devices.