Pharmaceutical Spotlight: Expanding Testing Capability for Drug Delivery Devices
In the world of pharmaceutical contract testing and manufacturing, supporting clients’ needs — both current and potential — is necessary to differentiate yourself in a crowded marketplace. Further, organizations must anticipate those changing customer needs and provide additional test services that address shifting demands.
Looking specifically at the injectable drug delivery space, patient expectations and innovations in drug formulations are both driving innovation and resulting in the use of more complex delivery mechanisms such as safety syringe devices and autoinjectors. Formulation and device teams within pharmaceutical organizations are more connected than ever, considering the full scope of the patient’s experience when creating and marketing combination products.
The testing requirements for these patient-centric devices are more intensive considering they include additional features and passive mechanisms that need to be evaluated. For example, as you compare a pre-filled syringe and an autoinjector, there are 3–4 additional Essential Drug Delivery Outputs, or EDDOs, to measure, utilizing additional measurement systems.
The increased volume of testing and capability gaps can cause bottlenecks, and in many cases, the burden will fall on contract organizations to support early-stage device evaluations and potentially even design verification.
For the contract organizations, there is also a benefit, as supporting these higher-value products can often demand premiums for the services. For many labs, this additional capability could only require additional fixture or system add-ons.
Pre-Filled Syringes and Safety Syringes
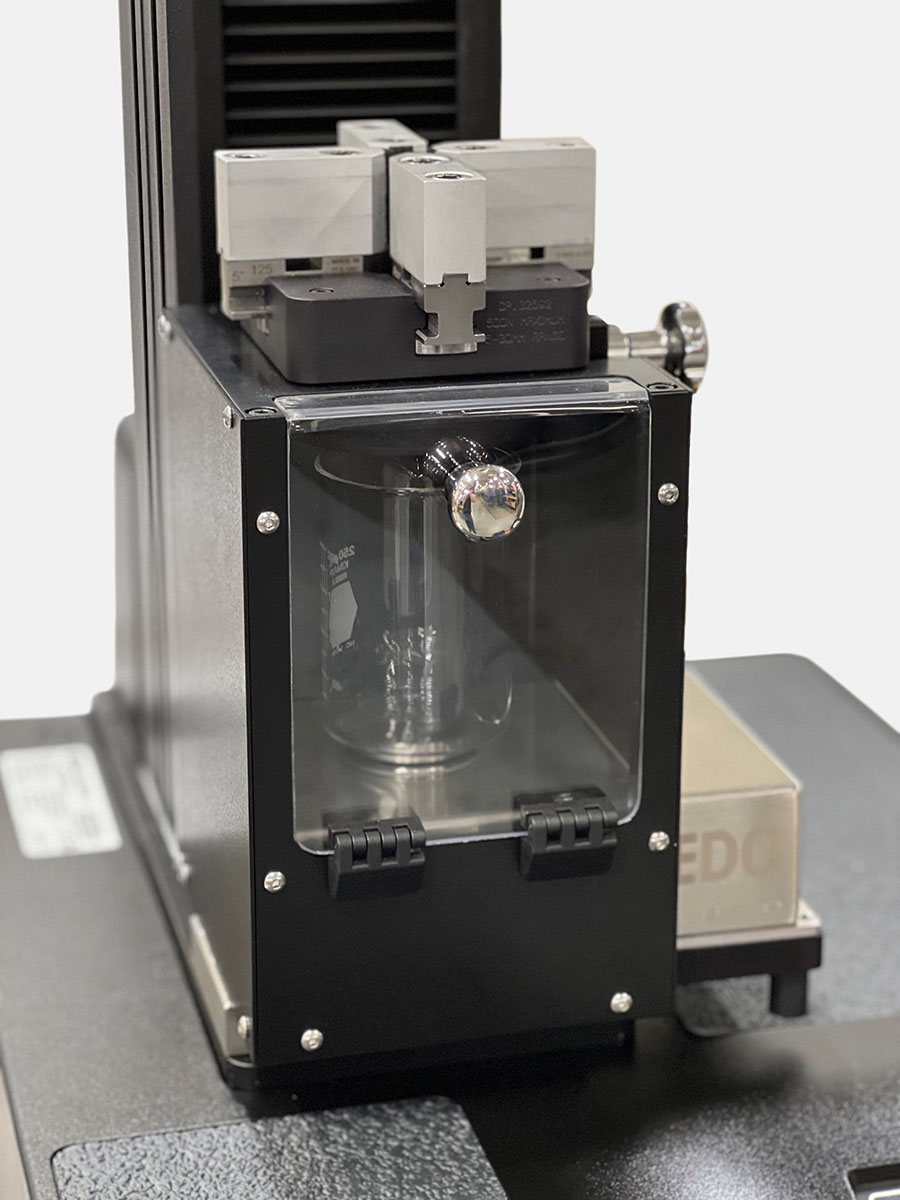
Many labs currently can perform basic break loose glide force testing on a range of syringe sizes. ISO 11040-8 discusses the requirements around measuring the delivered volume from the pre-filled syringe (PFS), and integrating a precision balance on the system is a simple way to improve the test process. Doing so can remove the manual process of moving the collection beaker to scale, limiting the amount of evaporation before taking the reading and ensuring all the data is captured in a single place.
Pre-filled syringes are often used as the primary container for autoinjector forces. When evaluating a PFS for use in an autoinjector, there are additional considerations that may warrant testing to additional annexes of ISO 11040-4. In the body of autoinjectors, the flange of the syringe takes the entirety of the reaction force from the device activation. These forces can be increasingly high, as more high-viscosity large molecule assets are developed. A modular fixture to allow testing of additional annexes of ISO 11040-4 such as flange breakage force can be useful to better characterize these devices.
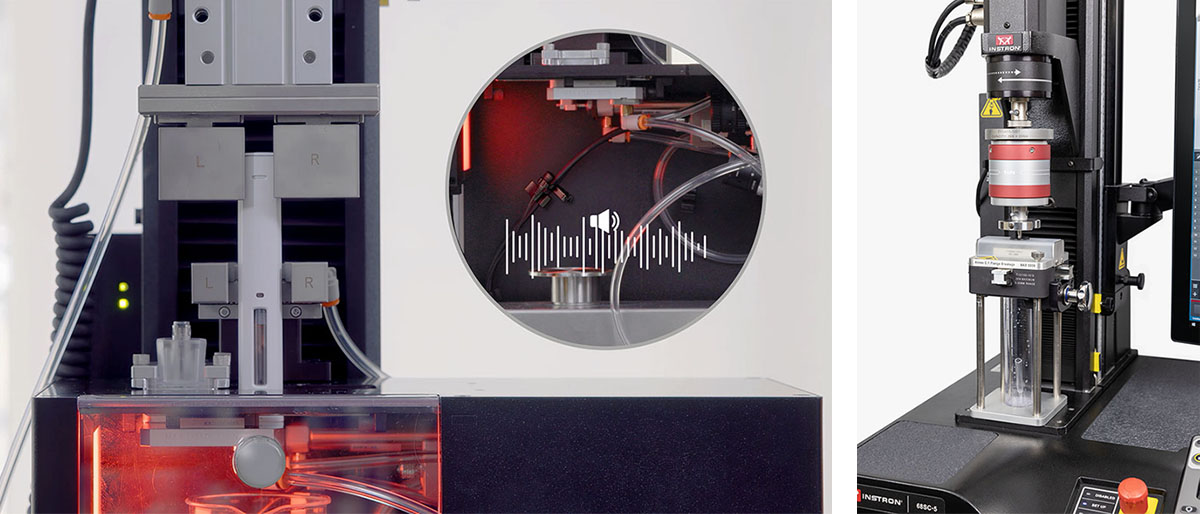
As more devices are being marketed for use directly by the patient, feedback is often built into the device based on human factors testing. This could take the form of auditory, visual, or tactile feedback for the patient. Auditory clicks are commonly used to indicate when the injection begins and when it ends. The visual window showing the drug is often referenced in the instructions for use, or IFU, for patients to refer to as an additional confirmation of a complete injection.
Integrating additional sensors into a system can provide a way to measure this feedback or at the very least record its occurrence to verify device performance. Click detection can be added to a system to capture real-time sound data superimposed on both force and mass profiles to fully evaluate the injection. Cameras can be added to integrate with the software and allow recording of the drug window, which can save with the test data as well as be reviewed by selecting a specific time stamp on the measurement profile to see the corresponding frame.
In partnership with pharmaceutical companies, device manufacturers, and CDMOs, Instron has developed a portfolio of fixturing and bespoke systems for testing devices and components in accordance with the following standards.
- ISO 11040-4
- ISO 11040-8
- ISO 11608-1,5,6
- ISO 80369-20
- USP 382, 1382
- ISO 8357
If you have any specific needs, please reach out to discuss how we can support expanding your test capability.