
TESTES BIOMÉDICOS
A Instron® traz uma vasta gama de conhecimentos para o setor biomédico, servindo como um parceiro estratégico para empresas de todos os portes. Nossos equipamentos e serviços são preparados para ajudá-lo a investigar novas tecnologias e garantir a qualidade do produto, tudo isso mantendo os mais altos níveis de integridade e segurança de dados. A Instron está inserida no setor biomédico há mais de 75 anos, e os desafios tecnológicos, regulatórios e de fabricação enfrentados por nossos clientes nos impulsionaram a desenvolver produtos e serviços para atender às suas necessidades. Esses desenvolvimentos incluem fixação especializada, software compatível e recursos de automação. Nosso ativo mais valioso é nossa extensa rede de clientes, que representa uma ampla gama de fabricantes de dispositivos médicos e farmacêuticos, CDMOs, universidades, laboratórios de testes e startups.
Nossos clientes estão na vanguarda da inovação tecnológica médica, trabalhando para melhorar os resultados dos pacientes, desenvolvendo produtos novos e melhores. Essas empresas estão aumentando a eficácia de tratamentos minimamente invasivos, desenvolvendo a próxima geração de sensores vestíveis e reimaginando um futuro onde robôs cirúrgicos são comuns no centro cirúrgico. Em cada caso, a Instron ajudou a construir um programa de testes mecânicos robusto, capaz de atender aos requisitos e superar os obstáculos de cada etapa do processo de desenvolvimento do produto.
A gama de aplicações biomédicas abrange uma vasta gama de áreas terapêuticas, trazendo consigo uma gama extremamente ampla de requisitos de teste. Vários arranjos de fixação devem ser adicionados a um sistema de teste universal para segurar ou manipular adequadamente os corpos de prova e obter os resultados necessários. Em muitos casos, as normas internacionais, como ISO ou ASTM, impulsionam o projeto final dessas fixações. Alternativamente, muitas são projetadas sob medida especificamente para as especificações do dispositivo do cliente. Nossa experiência no setor ajudou a criar um catálogo de garras e fixações para acomodar os dispositivos mais comuns e atender aos padrões mais frequentemente usados. Nosso Grupo de Soluções de Engenharia está disponível para trabalhar com você para desenvolver fixações específicas para suas necessidades.


APLICAÇÃO EM ALTA
TESTE DE DISPOSITIVOS VESTÍVEIS
As tendências na tecnologia vestível seguem as dos setores biomédico e eletrônico mais amplos – os dispositivos estão ficando menores, mais inteligentes e mais fáceis de usar. Os vestíveis na área da saúde evoluíram para soluções que reduzem o perfil do dispositivo, fornecem mais integração com aplicativos de smartphone e, o mais importante, permitem que os pacientes recebam seus tratamentos em casa, em vez de no consultório médico. À medida que essa tendência continua, os fabricantes estão trabalhando para desenvolver métodos de teste robustos para avaliar mecanicamente todos os aspectos desses dispositivos e garantir que eles estejam funcionando como esperado. Além de testar os componentes do dispositivo de injeção, os fabricantes também enfrentam desafios na avaliação e seleção dos adesivos para esses produtos.
O setor farmacêutico depende de testes mecânicos para avaliar os sistemas de administração de medicamentos e suas embalagens associadas. Os sistemas de administração de medicamentos podem utilizar vias dérmica, subcutânea, intramuscular, oral ou nasal e vêm em uma variedade de formatos de embalagem diferentes. Os sistemas de teste universais são usados ao longo do processo de desenvolvimento do produto para ajudar a identificar materiais adequados, avaliar mecanismos de administração, realizar validação de projeto, validar processos de fabricação e permitir medidas de CQ adequadas. As aplicações mais comuns estão relacionadas a sistemas de injeção baseados em agulhas e envolvem testes funcionais baseados em normas do setor, como ISO 11040 e ISO 11608, ou testes de usabilidade de produtos para complementar os testes de fatores humanos.
Os consumíveis médicos representam a maior subdivisão de testes biomédicos e incluem uma ampla variedade de produtos de uso único, como ferramentas cirúrgicas, EPI, produtos de fechamento de feridas, produtos de coleta de amostras e muito mais. Esses produtos são normalmente dispositivos médicos FDA classe I ou II, que, apesar de terem requisitos de teste menos rigorosos, são produzidos em quantidades tão grandes que é preciso ter cuidado para acomodar testes de alto volume. Para compensar os volumes maiores, a taxa de transferência e a repetibilidade tornam-se requisitos de teste críticos, abordados por meio de fixação especializada, fluxos de trabalho eficientes do operador e software intuitivo.
Tanto para fins de diagnóstico quanto terapêuticos, o mercado de dispositivos vasculares de intervenção cresceu exponencialmente. Produtos como fios-guia e cateteres são essenciais para o mapeamento da vasculatura, remoção de bloqueios e colocação de stents ou implantes. Avaliar as propriedades do material e do revestimento desses produtos é essencial para garantir que eles funcionarão como esperado in vivo. O teste in vitro também pode ser realizado usando sistemas turnkey construídos para imitar as condições do mundo real com modelos anatômicos, medindo as forças relacionadas à implantação e remoção desses dispositivos. Dispositivos implantados, como válvulas de substituição e stents, também são testados quanto à durabilidade de longo prazo usando sistemas dinâmicos para validar as respostas de longo prazo desses produtos às condições corporais.
Os biomateriais incluem os materiais encontrados na natureza, no corpo humano e em outras espécies animais. Esses materiais podem ser tecidos duros, como osso ou esmalte dentário, ou tecidos moles, como tendões e ligamentos. A variação biológica e os fatores ambientais afetam as propriedades mecânicas desses materiais. Eles também são anisotrópicos e não homogêneos, tornando-os materiais desafiadores para recriar ou projetar fora da natureza.
Implantes ortopédicos são implantes que sustentam o sistema esquelético. Estes incluem parafusos ósseos, placas, hastes e pinos para reparo de fraturas, bem como quadris, joelhos e componentes espinhais artificiais inteiros. Os implantes ortopédicos podem ser inseridos temporariamente no corpo para auxiliar na cicatrização de um paciente ou podem ser inseridos no corpo com a intenção de que o implante sobreviva ao paciente. Dependendo do seu uso, os implantes ortopédicos são normalmente considerados dispositivos médicos Classe II ou Classe III pela FDA e exigem uma gama e combinação de testes mecânicos estáticos e de fadiga.
Os materiais odontológicos são normalmente compostos de metal, elastômeros e polímeros. Dispositivos restauradores e protéticos são frequentemente compostos de vários materiais que exigem testes mecânicos para determinar como esses materiais interagem para formar o dispositivo acabado. Um implante dentário é um poste de metal, normalmente titânio, que substitui todo o dente do paciente. O teste de fadiga representa a forma mais comum de teste mecânico realizado em implantes dentários, seguindo as normas internacionais para avaliar o desgaste esperado do uso repetido.
INTEGRIDADE E VALIDAÇÃO DE DADOS
O conceito de integridade de dados é fundamental para a fabricação de produtos biomédicos e impulsiona os programas de qualidade que garantem a segurança do produto. Globalmente, existem inúmeros arcabouços regulatórios que visam definir o que é integridade de dados e como ela deve ser garantida, seja ditada pela FDA, GMP ou outros órgãos nacionais ou internacionais.
Conformidade regulatória
Dentro do setor biomédico, existem órgãos regulatórios nacionais e internacionais que definem os protocolos necessários para garantir que a segurança do paciente esteja no centro do desenvolvimento e fabricação do produto. Organizações como a FDA e a EU MDR exigem processos rigorosos de controle de qualidade e integridade de dados. Nosso conjunto de softwares e produtos de serviço permite que os clientes mantenham melhor a conformidade para seus programas de teste mecânico.
Serviços de Validação IQOQ
Nossas equipes de serviço de campo fornecem serviços de validação e documentação para apoiar os processos de IQOQ projetados para garantir que seu equipamento de teste Instron funcione conforme seus propósitos pretendidos e produza resultados válidos de acordo com o 21 CFR 820.72 e ISO 13845. Ao final de nossos serviços, fornecemos um Certificado de Conclusão para Qualificação de Instalação e Operação que será assinado pelo Engenheiro de Serviço de Campo da Instron que realizou as validações.
Bluehill Central
O software Bluehill Central é uma ferramenta de gerenciamento laboratorial que permite o gerenciamento centralizado e remoto dos aplicativos do software Bluehill Universal associados a múltiplas estruturas de teste Instron. O software permite gerenciar remotamente todos os usuários do Bluehill Universal, modelos de teste, resultados, aprovações de revisão de arquivos e dados de trilha de auditoria de múltiplos sistemas Instron.
Rastreabilidade
O Módulo de Rastreabilidade do Bluehill Universal permite que os usuários atendam aos requisitos de auditoria associados ao FDA 21 CFR Parte 11, bem como aos da ISO 17025, Nadcap e outros órgãos regulatórios. Por meio da integração perfeita de aprovações eletrônicas, histórico de revisão e uma trilha de auditoria automatizada, este poderoso complemento se combina com a segurança integrada do Bluehill para fornecer rastreabilidade de dados incomparável.
Saiba mais sobre a rastreabilidade do Bluehill
Saiba mais sobre integridade de dados e rastreabilidade do Bluehill
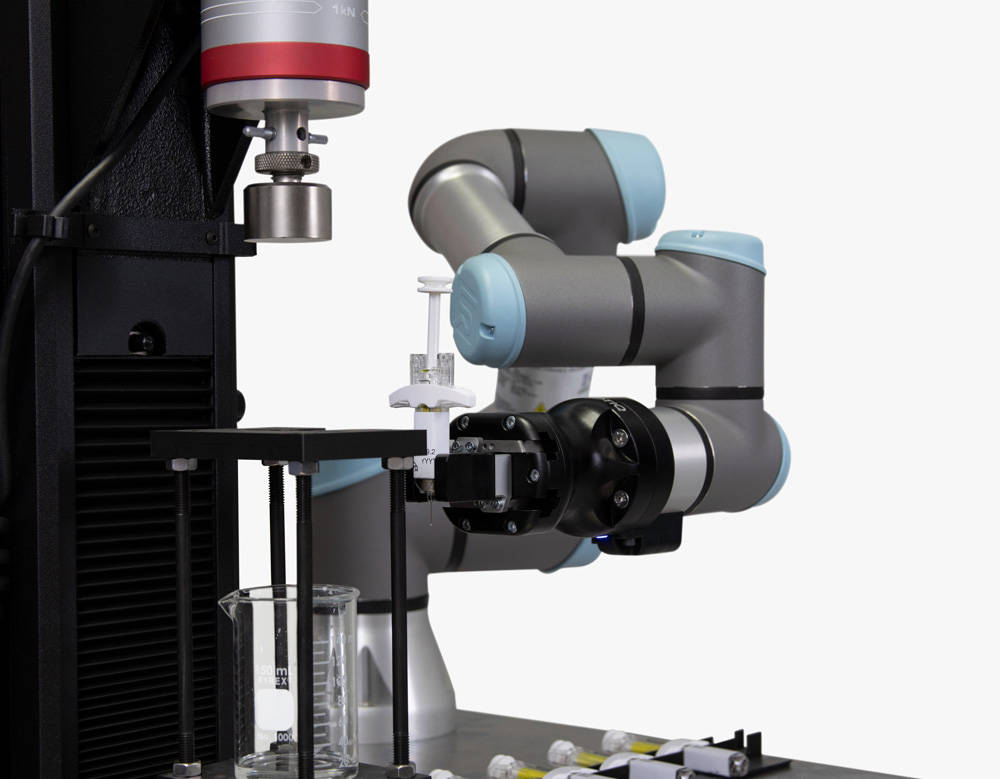
A automação representa uma oportunidade de crescimento em uma variedade de aplicações e em todo o processo de desenvolvimento do produto. Em laboratórios de CQ de pequeno e médio volume, as dificuldades de rotatividade de operadores e dificuldades de contratação estão impulsionando a adoção de tecnologia de automação, como cobots e estágios XY para melhorar a eficiência do laboratório. Mesmo dentro de laboratórios de P&D ou verificação de dispositivos, a automação pode garantir que os engenheiros de teste e outros funcionários qualificados possam concentrar seu tempo em atividades de valor agregado, em vez de testes manuais. À medida que as empresas avançam para a produção de dispositivos, alcançar o teste mecânico completo em linha é uma alavanca crítica para melhorar o controle do processo de fabricação e reduzir o desperdício de material ou produtos defeituosos. Cada instância de adição de automação tem o potencial de melhorar a eficiência do laboratório, reduzir a variabilidade e simplificar os procedimentos de teste.
SISTEMAS DE ENSAIO RECOMENDADOS







 Teste ISO 7886-1 de seringas hipodérmicas estéreis
Teste ISO 7886-1 de seringas hipodérmicas estéreis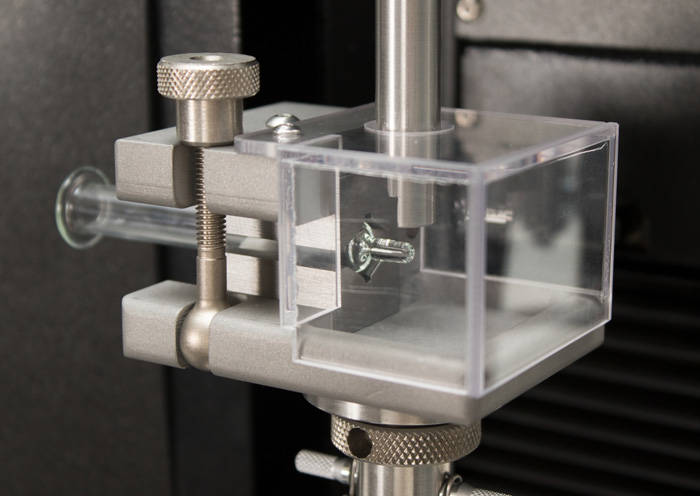 ISO 11040 - Propriedades de design e funcionais de seringas pré-carregadas
ISO 11040 - Propriedades de design e funcionais de seringas pré-carregadas ISO 80369 - Conectores de pequeno diâmetro para líquidos
ISO 80369 - Conectores de pequeno diâmetro para líquidos Teste de agulha de seringa
Teste de agulha de seringa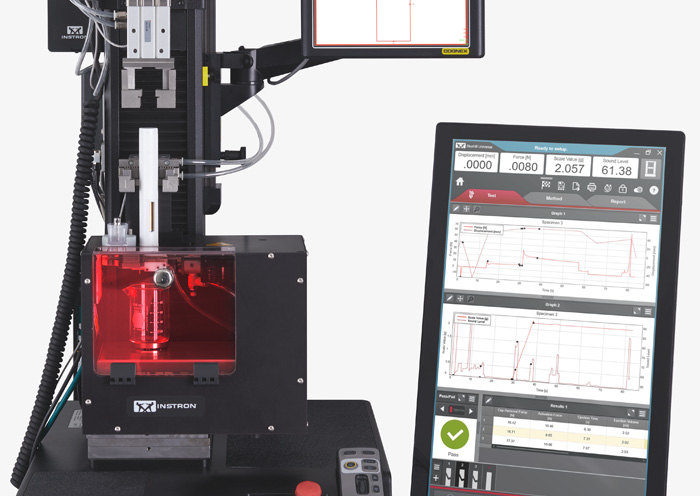 ISO 11608-1:2022 Sistemas de injeção baseados em agulhas
ISO 11608-1:2022 Sistemas de injeção baseados em agulhas Teste de força de vedação residual (RSF)
Teste de força de vedação residual (RSF) Desempenho de impacto de comprimidos farmacêuticos
Desempenho de impacto de comprimidos farmacêuticos ASTM D7860 Retenção de torque para embalagens resistentes a crianças
ASTM D7860 Retenção de torque para embalagens resistentes a crianças Teste de EPI - incluindo máscaras, luvas e cotonetes nasais
Teste de EPI - incluindo máscaras, luvas e cotonetes nasais ASTM F88 Resistência da vedação de materiais de barreira flexíveis
ASTM F88 Resistência da vedação de materiais de barreira flexíveis Teste de agulha curva de acordo com a ASTM F3014
Teste de agulha curva de acordo com a ASTM F3014 EN455-2, ISO 11193, ASTM D6319 Teste de tração de luvas médicas
EN455-2, ISO 11193, ASTM D6319 Teste de tração de luvas médicas Teste de tração de suturas cirúrgicas
Teste de tração de suturas cirúrgicas Teste de stent
Teste de stent Teste de fadiga de materiais e estruturas de stent
Teste de fadiga de materiais e estruturas de stent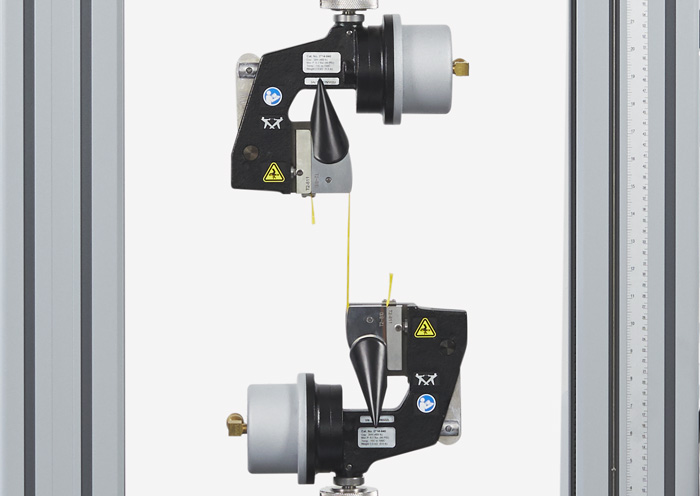 Teste de tração ISO 10555 de tubos de cateter
Teste de tração ISO 10555 de tubos de cateter Teste de fio-guia
Teste de fio-guia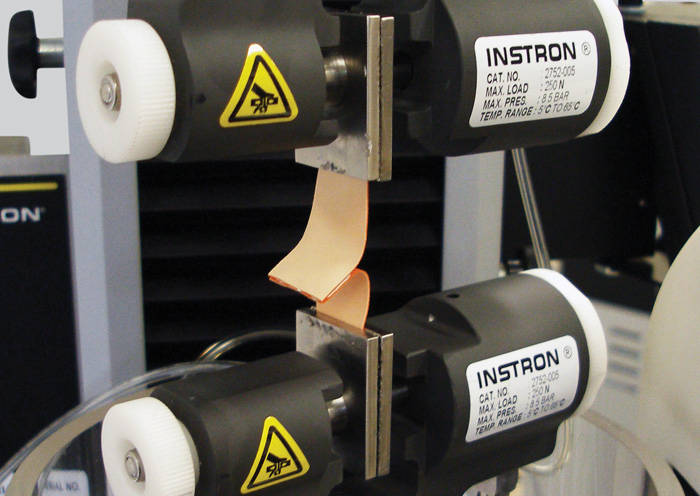 ASTM F2458 Resistência ao fechamento de feridas de adesivos e selantes de tecido
ASTM F2458 Resistência ao fechamento de feridas de adesivos e selantes de tecido ASTM F2256 Propriedades de resistência de adesivos de tecido por teste de descascamento em T
ASTM F2256 Propriedades de resistência de adesivos de tecido por teste de descascamento em T Teste de hidrogéis de polímero para ASTM F2150
Teste de hidrogéis de polímero para ASTM F2150 Teste de tecido duro
Teste de tecido duro Teste de tecido mole
Teste de tecido mole ASTM F543 - Teste axial e torcional de parafusos ósseos médicos metálicos
ASTM F543 - Teste axial e torcional de parafusos ósseos médicos metálicos Teste de fadiga cíclica de construções de implantes espinhais de acordo com ASTM F1717-18, ASTM F2706-18 e ISO 12189-8
Teste de fadiga cíclica de construções de implantes espinhais de acordo com ASTM F1717-18, ASTM F2706-18 e ISO 12189-8 Teste de fadiga cíclica de próteses de implante de quadril artificial de acordo com ISO 7206-4, ISO 7206-6, ISO-7206-8 e ASTM F2068
Teste de fadiga cíclica de próteses de implante de quadril artificial de acordo com ISO 7206-4, ISO 7206-6, ISO-7206-8 e ASTM F2068 Teste de dispositivo de fixação de fratura
Teste de dispositivo de fixação de fratura ASTM F2077 Caracterização e fadiga de dispositivos de fusão de corpo intervertebral espinhal
ASTM F2077 Caracterização e fadiga de dispositivos de fusão de corpo intervertebral espinhal ASTM F2267 Avaliação de dispositivos de fusão de corpo intervertebral espinhal sob carga axial
ASTM F2267 Avaliação de dispositivos de fusão de corpo intervertebral espinhal sob carga axial Teste de fadiga cíclica de componentes de bandeja tibial de acordo com ASTM F1800 e ISO 14879
Teste de fadiga cíclica de componentes de bandeja tibial de acordo com ASTM F1800 e ISO 14879 ISO 16402 Teste de flexão de cimentos de resina acrílica usados em ortopedia
ISO 16402 Teste de flexão de cimentos de resina acrílica usados em ortopedia Teste de fadiga ISO 14801 de implantes dentários endósseos pré-angulados em banho de fluido
Teste de fadiga ISO 14801 de implantes dentários endósseos pré-angulados em banho de fluido Teste de aparelhos ortodônticos: materiais de metal, plástico e cerâmica
Teste de aparelhos ortodônticos: materiais de metal, plástico e cerâmica









