Determining the Residual Seal Force (RSF)
MECHANICAL TESTING OF MEDICAL VIALS

Parenteral products such as injectable medications and vaccines are commonly packaged in glass vials. These vials must maintain a robust seal between the glass vial and the elastomeric closure to prevent product contamination and leakage. Residual Seal Force (RSF) is an evaluation of the quality of this seal.
The glass vials subjected to RSF evaluation are used exclusively within the healthcare industry. A perfect example of the importance of RSF testing is the COVID-19 vaccine: some of the vaccines need to be stored at extremely cold temperatures in order to remain effective. Extremely cold temperatures can cause elastomeric materials to contract, which could cause the vial seals to leak. RSF testing can be performed at the full range of possible temperatures to assist manufacturers in evaluating raw materials and assembly processes. This verification is critical in ensuring that products can withstand travel and storage before being administered to the patient.
"Residual s eal force" refers to the force that the elastomeric seal is placing on the aluminum cap of the vial. This force measurement indicates the security of the vial closure. The seal consists of a rubber stopper and an aluminum cap that is crimped onto the vial with an initial force. Over time, this force declines due to the relaxation behavior of rubber, a decay of the force imposed by the cap, and various processing procedures.
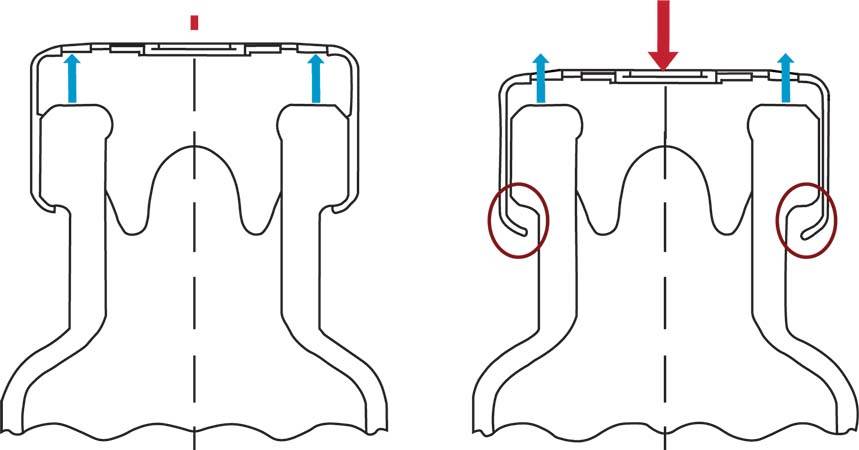
Determining the Residual Seal Force
The United States Pharmacopeia (USP) outlines standards for the determination of Container Closure Integrity (CCI), a measure of the ability of a container to prevent leakage through non-destructive means. Many studies have shown a correlation between residual seal force values and the CCI performance of the container, prompting many vial manufacturers to adopt RSF testing into their quality program. Residual seal force results can also be correlated to the capping and crimping process, helping manufacturers optimize their cap application settings to maximize CCI.
Materials Testing System
RSF testing can be performed on a low force Instron system such as a 68SC1 with a 500 N or 1,000 N 2580 Series load cell. Instron also offers a specialized fixture (CP131410) designed to exert a compressive load around the edge of a vial until the lower lip of the aluminum cap breaks away and begins to move in a direction opposite to the loading. The top compressive fixture is spherically seated, which allows for self-pivoting in order to maintain near perfect alignment, even if the cap itself is misaligned. Automatic measurement of the residual seal force requires software capable of measuring the decline in load associated with the sealing process. The force where the aluminum cap breaks away from the top and moves in the direction opposite to loading is visible in the software as a plateau seen in the graph. The residual seal force can be calculated using either a manual cursor select or an automatic calculation in Bluehill Universal software.
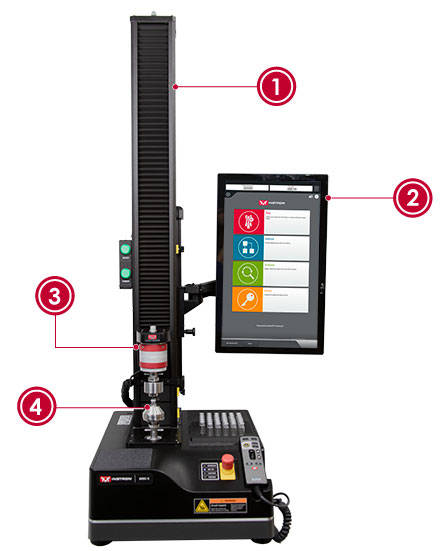
| RSF Test Setup | |
|---|---|
| 1) | 6800 Series Universal Testing System |
| 2) | Bluehill Universal Software |
| 3) | 2580 Series Load Cell |
| 4) | Custom Test Fixture CP131410 |
Testing Tips and Tricks
- Reducing variability when testing residual seal force can be difficult to control. Since elastomeric vial seals relax in the short term and age in the long term, it is important to make sure that all specimens in a sample are capped at approximately the same time to minimize variability during testing. Alignment is also critical for repeatable results. Fixturing should help the operator maintain concentricity between the vial and the platen surface, as off-center loading can greatly increase the variability between tests.
- By placing a compressive load on the vial cap, a clear inflection point will appear in the curve at the point where the RSF point has been surpassed causing separation of the crimped cap from the head of the vial.
- To learn more about mechanical testing for drug delivery devices, click here.


