ISO 80369 Small-Bore Connectors for Liquids and Gases in Healthcare Applications
Mechanical Testing Requirements for Luer Connectors per ISO 80369
Written By: Landon Goldfarb
Updated: November 14, 2025
ISO 80369 is an international standard that specifies the testing requirements for small-bore connectors used in medical devices. Its primary goal is to prevent misconnections and ensure patient safety. The standard specifies mechanical tests that verify the safety and reliability of the connector under clinical conditions.
These tests include:
- Axial pull-out force
- Leakage resistance
- Stress cracking
- Dimensional verification
For manufacturers and contract test labs, understanding these protocols is essential for compliance and product integrity across connector types, including
- Enteral
- Respiratory
- Limb cuff inflation
- Neural
- Intravascular or hypodermic
Overview of Luer Connectors
Luer connectors are small-bore connectors that are universally used across medical applications to ensure compatibility between components like syringes and catheters. They enable reliable connections regardless of the manufacturer, supporting critical functions such as medication delivery and bodily fluid withdrawal.
Failures in these connectors can lead to dangerous complications, including:
- Contamination of medication
- Inability to make a secure connection between devices
Because of their widespread use, ISO 80369 is one of the most frequently tested standards in the medical device industry.
Types of Luer Connectors
Luer connectors can be male or female and come in two types:
- Luer Slips: Caps held in place by the frictional forces introduced by a 6° tapered fitting
- Luer Locks: Caps with a threaded connection, offering a more secure attachment
The type of Luer connector affects:
- Test requirements
- Expected results
- Interface specifications for reference connectors
Reference connectors are specified in ISO 80369 with precise dimensions and tolerances for the mating connections to the device under test.
Testing to ISO 80369
Parts of the Standard
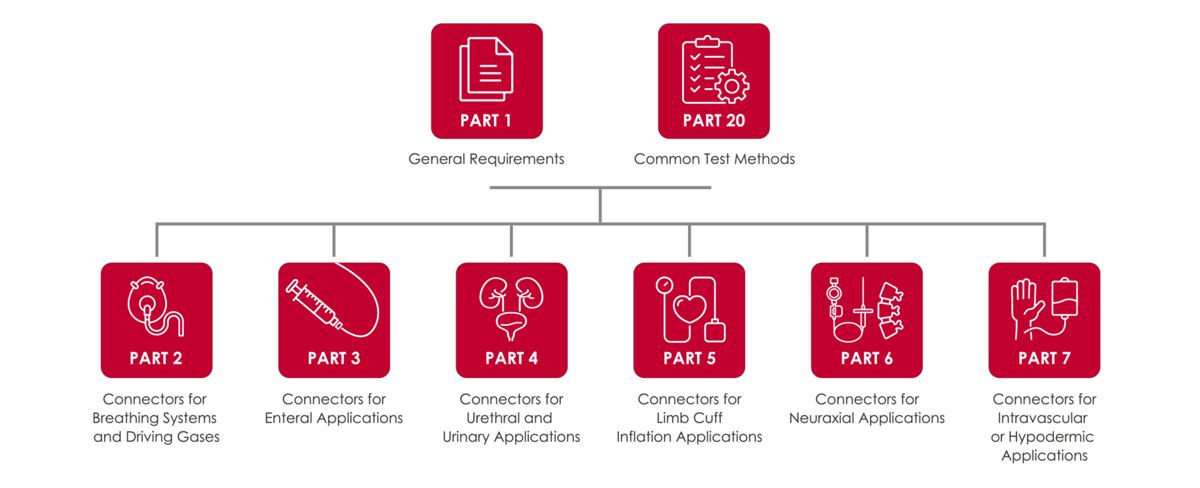
ISO 80369 includes eight parts, each addressing specific applications and test methods:
- Part 1: General Requirements
- Part 2: Connectors for Breathing Systems and Driving Gases
- Part 3: Connectors for Enteral Applications
- Part 4: Connectors for Urethral and Urinary Applications
- Part 5: Connectors for Limb Cuff Inflation Applications
- Part 6: Connectors for Neuraxial Applications
- Part 7: Connectors for Intravascular or Hypodermic Applications
- Part 20: Common Test Methods
Part 1 outlines general requirements and background for Luer connectors and their applications.
Parts 2 through 7 include application specific details, such as connector geometry and testing criteria.
Part 20 includes the complete procedures for each test defined by the standard. It also provides alternative methods for obtaining variable data – as opposed to attribute data - effectively reducing the required sample size.
This modification changes the test results from a qualitative assessment – whether leakage or damage was visible – to a quantitative value. This change can require the device to be tested to failure, requiring higher forces and torques.
In practice, the number of test devices may be limited – as is often the case for product design verification. Using variable data can be helpful in these circumstances.
Key Testing Annexes
Nine testing annexes define how to evaluate closure quality. The tests simulate typical and extraordinary use cases using applied forces, torques, and internal pressures.
- Annex B: Leakage by pressure decay
- Annex C: Falling drop positive-pressure liquid leakage
- Annex D: Subatmospheric-pressure air leakage
- Annex E: Stress Cracking
- Annex F: Resistance to separation from axial load
- Annex G: Resistance to separation from unscrewing
- Annex H: Resistance to overriding
- Annex I: Disconnection by unscrewing
- Annex K: Air leakage during aspiration (new to the 2024 version of the standard)
Challenges in ISO 80369 Testing
- Multiple Test Setups - Manual methods slow down throughput and require frequent equipment changes. Integrating the pressure sensor and generator into the universal testing machine streamlines the workflow and saves lab space.
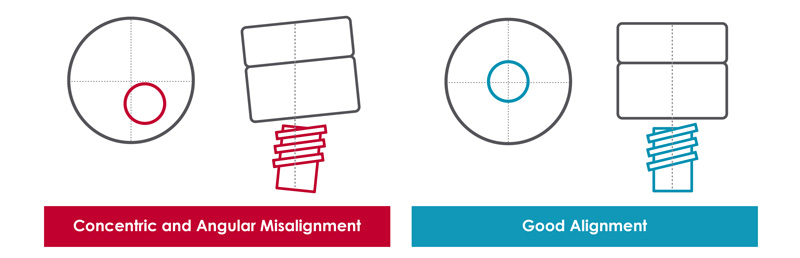
- System Alignment - Achieving the required assembly forces and torques depends on precise concentric and angular alignment. Misalignment can lead to non-compliant results. Using an XY stage to reduce misalignment helps to ensure reliable data.
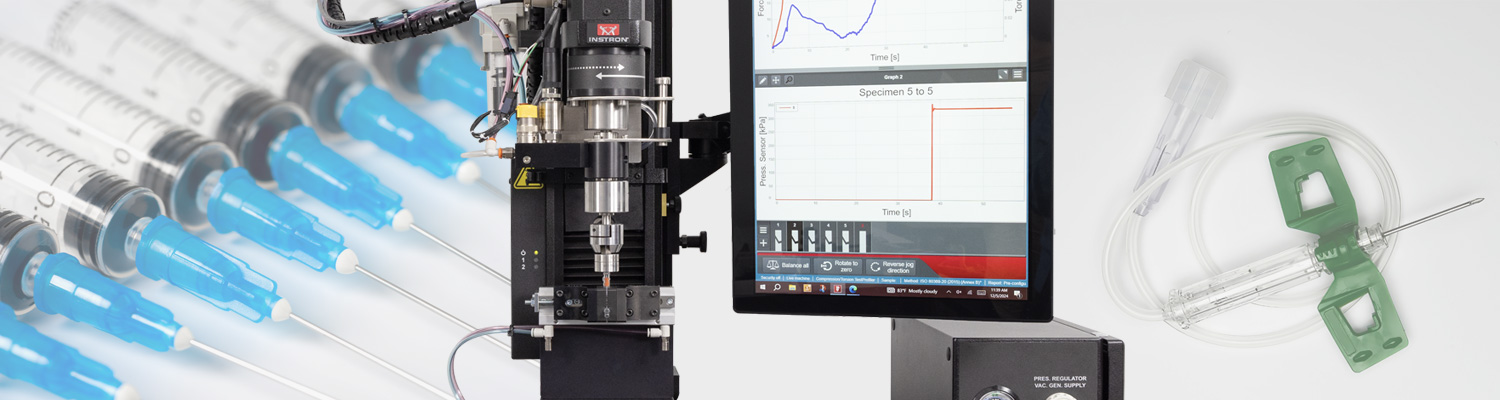
Recommended Materials Testing System
For ISO 80369 compliance, we recommend the Instron 68SC-1 universal testing system with the Torsion Add-On 3.0. This configuration enables biaxial testing – combining axial and torsional forces – to evaluate the mechanical performance of Luer connectors.
Features of the 68SC-1 system include:
- Configurations for positive and negative pressure testing
- Interchangeable fixtures for syringes and custom hubs and connectors
- Modular design for testing a wide range of devices
Instron's ISO 80369 testing system support Annexes B through I, providing the required flexibility to support testing of syringes, IV hubs, connectors, and more.

Test Setup for ISO 80369
- Reference Connector
- Device Specific Fixturing
- Manual XY Stage
- Biaxial Load Cell
- Torsion Add-On
- Pressure Controller
Summary
ISO 80369 ensures the safety and reliability of small-bore connectors in healthcare. Compliance requires understanding connector types, test methods, and equipment capabilities. By optimizing workflows and using advanced testing systems, labs can improve efficiency and accuracy.
To ensure compliance, always read the full ISO 80369 standard.
Visit our guide for syringe, cartridge, and vial testing to learn more.



