
BIOMEDICAL TESTING
Instron® brings a wealth of knowledge to the biomedical industry, serving as a strategic partner to companies of all sizes. Our equipment and services are primed to help you investigate new technologies and ensure product quality, all while maintaining the highest levels of data integrity and security. Instron has been embedded in the biomedical industry for more than 75 years, and the technological, regulatory, and manufacturing challenges facing our customers have driven us to develop products and services to address their needs. These developments include specialized fixturing, compliant software, and automation capabilities. Our most valuable asset is our extensive customer network, which represents a wide range of medical device and pharmaceutical manufacturers, CDMOs, universities, test houses, and startups.
Our customers are on the forefront of medical technological innovation, working to improve patient outcomes by developing new and better products. These companies are increasing the efficacy of minimally invasive treatments, developing the next generation of wearable sensors, and reimagining a future where surgical robots are commonplace in the operating room. In each case, Instron has helped build a robust mechanical testing program able to meet the requirements and overcome the hurdles of each step of the product development process.
The range of biomedical applications spans a vast array of therapeutic areas, bringing with it an extremely broad range of testing requirements. Various fixturing arrangements must be added to a universal testing system to properly hold or manipulate the test specimens and attain the necessary results. In many cases, international standards such as ISO or ASTM drive the ultimate design of these fixtures. Alternatively, many are custom designed specifically to the customer's device specifications. Our experience within the industry has helped create a catalog of grips and fixtures to accommodate the most common devices and meet the most frequently used standards. Our Engineered Solution Group is available to work with you to develop fixtures specific to your needs.


TRENDING APPLICATION
WEARABLE DEVICE TESTING
Trends in wearable technology follow those of the broader biomedical and electronics industries – devices are getting smaller, smarter, and easier to use. Wearables in healthcare have moved toward solutions that reduce the device profile, provide more integration with smartphone apps, and most importantly enable patients to receive their treatments at home instead of in a doctor's office. As this trend continues, manufacturers are working to develop robust test methods to mechanically evaluate all aspects of these devices and ensure they are performing as expected. In addition to testing the injection device components, manufacturers also face challenges in evaluating and selecting the adhesives for these products.
The pharmaceutical industry relies on mechanical testing to evaluate drug delivery systems and their associated packaging. Drug delivery systems can utilize dermal, subcutaneous, intramuscular, oral, or nasal routes and come in a variety of different packaging formats. Universal testing systems are used throughout the product development process to help identify suitable materials, evaluate delivery mechanisms, perform design validation, validate manufacturing processes, and enable proper QC measures. The most common applications are related to needle based injection systems and involve either functional testing based on industry standards such as ISO 11040 and ISO 11608 or usability testing of products to supplement human factor testing.
Medical consumables represent the largest subsection of biomedical testing and include a wide variety of single-use products such as surgical tools, PPE, wound closure products, specimen collection products, and more. These products are typically either FDA class I or II medical devices, which, despite having less stringent test requirements, are produced in such large quantities that care must be taken to accommodate high volume testing. To compensate for the larger volumes, throughput and repeatability become critical test requirements, addressed through specialized fixturing, efficient operator workflows, and intuitive software.
For both diagnostic and therapeutic purposes, the market for interventional vascular devices has grown exponentially. Products such as guidewires and catheters are essential for the mapping of vasculature, removal of blockages, and placement of stents or implants. Assessing the material and coating properties of these products is essential for ensuring they will perform as expected in vivo. Testing in vitro can also be accomplished using turnkey systems built to mimic real-world conditions with anatomical models, measuring the forces related to the deployment and removal of these devices. Implanted devices such as replacement valves and stents are also tested for long-term durability using dynamic systems to validate the long-term responses of these products to body conditions.
Biomaterials include the materials found in nature, the human body, and other animal species. These materials can be hard tissues such as bone or dental enamel, or soft tissues such as tendons and ligaments. Biological variation and environmental factors affect the mechanical properties of these materials. They are also anisotropic and nonhomogeneous, making them challenging materials to re-create or engineer outside of nature.
Orthopedic implants are implants that support the skeletal system. These include bone screws, plates, rods and pins for fracture repair, as well as entire artificial hips, knees, and spinal components. Orthopedic implants can be temporarily inserted into the body to assists a patient’s healing, or can be inserted into the body with the intent that the implant will outlive the patient. Depending on their use, orthopedic implants are typically considered to be Class II or Class III medical devices by the FDA and require a range and combination of static and fatigue mechanical testing.
Dental materials are typically composed of metal, elastomers, and polymers. Restorative and prosthodontic devices are often composed of multiple materials that require mechanical testing to determine how these materials interact to form the finished device. A dental implant is a metal post, typically titanium, that replaces the patient’s entire tooth. Fatigue testing represents the most common form of mechanical testing performed on dental implants, following international standards to evaluate the expected wear of repeated use.
DATA INTEGRITY AND VALIDATION
The concept of data integrity is foundational to the manufacturing of biomedical products and drives the quality programs that ensure product safety. Globally, there are numerous regulatory frameworks that aim to define what data integrity is and how it should be ensured, whether dictated by the FDA, GMP, or other national or international bodies.
Regulatory Compliance
Within the biomedical industry there are both national and international regulatory bodies defining the protocols necessary to ensure that patient safety is at the center of product development and manufacturing. Organizations like the FDA and EU MDR demand stringent quality control and data integrity processes. Our suite of software and service products enable customers to better maintain compliance for their mechanical test programs.
IQOQ Validation Services
Our field service teams provide validation and documentation services to support IQOQ processes designed to ensure that your Instron testing equipment performs to its intended purposes and produces valid results per 21 CFR 820.72 and ISO 13845. At the conclusion of our services we provide a Completion Certificate for Installation and Operational Qualification that will be signed by the Instron Field Service Engineer who performed the validations.
Bluehill Central
Bluehill Central software is a laboratory management tool that enables centralized, remote management of Bluehill Universal software applications associated with multiple Instron test frames. The software allows you to remotely manage all Bluehill Universal users, test templates, results, file revision approvals, and audit trail data from multiple Instron systems.
Traceability
Bluehill Universal’s Traceability Module enables users to meet the audit requirements associated with FDA 21 CFR Part 11 as well as those of ISO 17025, Nadcap, and other regulatory bodies. Through seamless integration of electronic approvals, revision history, and an automated audit trail, this powerful add-on combines with Bluehill’s built-in security to provide unmatched data traceability.
Learn more about Bluehill Traceability
Learn more about data integrity and Bluehill Traceability
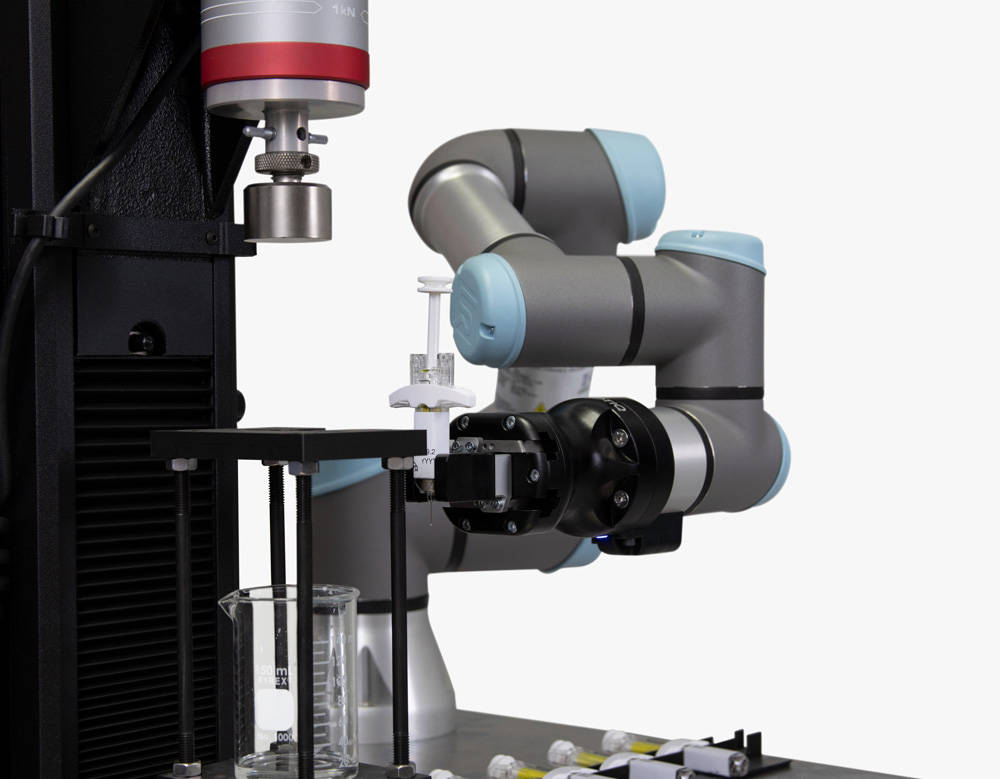
Automation represents an opportunity for growth across a range of applications and throughout the entire product development process. In small and mid volume QC labs, the pains of operator turnover and hiring difficulties are driving the adoption of automation technology such as cobots and XY stages to improve lab efficiency. Even within R&D or device verification labs, automation can ensure that test engineers and other skilled employees are able to focus their time on value added activities rather than manual testing. As companies move to device production, achieving full in-line mechanical testing is a critical lever for improving manufacturing process control and reducing material waste or faulty products. Each instance of adding automation has the potential to improve lab efficiency, reduce variability, and simplify test procedures.
RECOMMENDED TESTING SYSTEMS







 ISO 7886-1 Testing Sterile Hypodermic Syringes
ISO 7886-1 Testing Sterile Hypodermic Syringes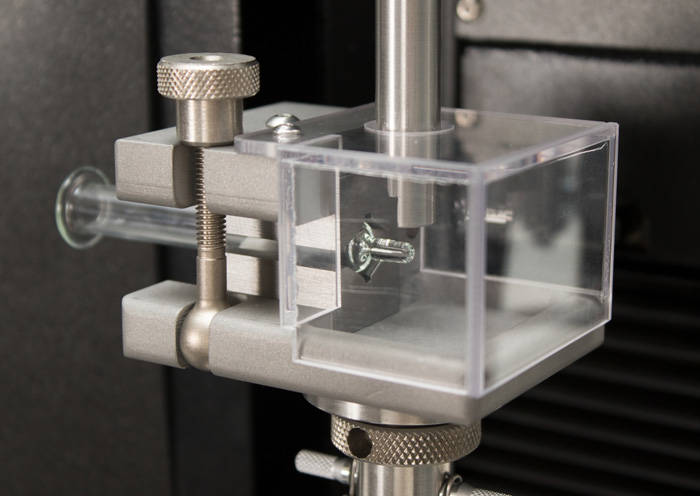 ISO 11040 - Design and Functional Properties of Prefilled Syringes
ISO 11040 - Design and Functional Properties of Prefilled Syringes ISO 80369 - Small Bore Connectors for Liquids
ISO 80369 - Small Bore Connectors for Liquids Syringe Needle Testing
Syringe Needle Testing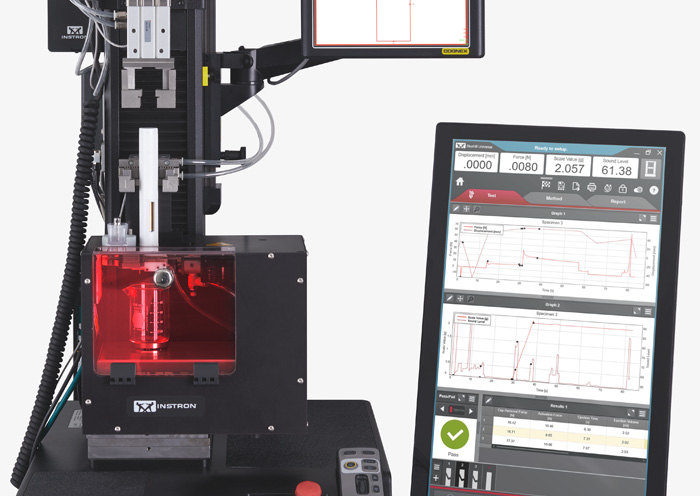 ISO 11608-1:2022 Needle-Based Injection Systems
ISO 11608-1:2022 Needle-Based Injection Systems Residual Seal Force (RSF) Testing
Residual Seal Force (RSF) Testing Impact Performance of Pharmaceutical Tablets
Impact Performance of Pharmaceutical Tablets ASTM D7860 Torque Retention for Child Resistant Packaging
ASTM D7860 Torque Retention for Child Resistant Packaging PPE Testing - Including Masks, Gloves, and Nasal Swabs
PPE Testing - Including Masks, Gloves, and Nasal Swabs ASTM F88 Seal Strength of Flexible Barrier Materials
ASTM F88 Seal Strength of Flexible Barrier Materials Curved Needle Testing to ASTM F3014
Curved Needle Testing to ASTM F3014 EN455-2, ISO 11193, ASTM D6319 Tensile Testing of Medical Gloves
EN455-2, ISO 11193, ASTM D6319 Tensile Testing of Medical Gloves Tensile Testing of Surgical Sutures
Tensile Testing of Surgical Sutures Stent Testing
Stent Testing Fatigue Testing Stent Materials and Structures
Fatigue Testing Stent Materials and Structures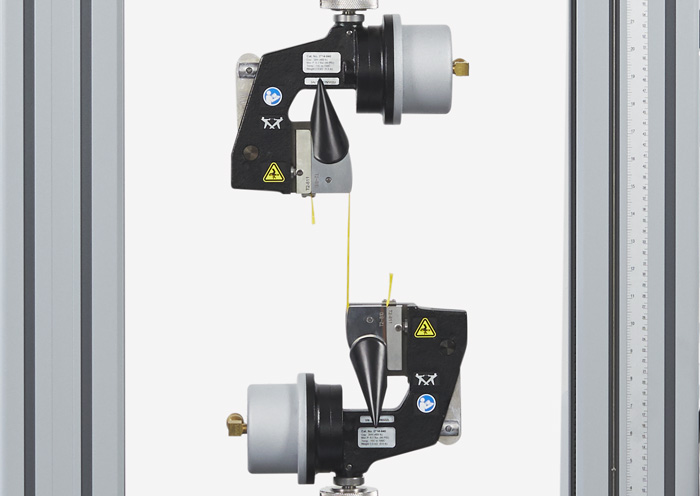 ISO 10555 Tensile Testing of Catheter Tubing
ISO 10555 Tensile Testing of Catheter Tubing Guidewire Testing
Guidewire Testing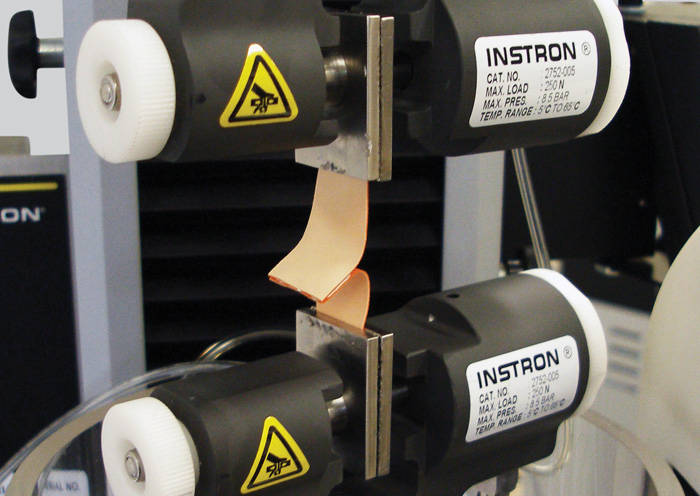 ASTM F2458 Wound Closure Strength of Tissue Adhesives and Sealants
ASTM F2458 Wound Closure Strength of Tissue Adhesives and Sealants ASTM F2256 Strength Properties of Tissue Adhesives by T-Peel Testing
ASTM F2256 Strength Properties of Tissue Adhesives by T-Peel Testing Testing Polymer Hydrogels to ASTM F2150
Testing Polymer Hydrogels to ASTM F2150 Hard Tissue Testing
Hard Tissue Testing Soft Tissue Testing
Soft Tissue Testing ASTM F543 - Axial and Torsional Testing of Metallic Medical Bone Screws
ASTM F543 - Axial and Torsional Testing of Metallic Medical Bone Screws Cyclic Fatigue Testing of Spinal Implant Constructs in accordance with ASTM F1717-18, ASTM F2706-18 and ISO 12189-8
Cyclic Fatigue Testing of Spinal Implant Constructs in accordance with ASTM F1717-18, ASTM F2706-18 and ISO 12189-8 Cyclic Fatigue Testing of Artificial Hip Implant Prostheses in accordance with ISO 7206-4, ISO 7206-6, ISO-7206-8 and ASTM F2068
Cyclic Fatigue Testing of Artificial Hip Implant Prostheses in accordance with ISO 7206-4, ISO 7206-6, ISO-7206-8 and ASTM F2068 Fracture Fixation Device Testing
Fracture Fixation Device Testing ASTM F2077 Characterization and Fatigue of Spinal Intervertebral Body Fusion Devices
ASTM F2077 Characterization and Fatigue of Spinal Intervertebral Body Fusion Devices ASTM F2267 Evaluating Spinal Intervertebral Body Fusion Devices Under Axial Loading
ASTM F2267 Evaluating Spinal Intervertebral Body Fusion Devices Under Axial Loading Cyclic Fatigue Testing of Tibial Tray Components in accordance with ASTM F1800 and ISO 14879
Cyclic Fatigue Testing of Tibial Tray Components in accordance with ASTM F1800 and ISO 14879 ISO 16402 Flexural Testing of Acrylic Resin Cements Used in Orthopedics
ISO 16402 Flexural Testing of Acrylic Resin Cements Used in Orthopedics ISO 14801 Fatigue Testing of Pre-Angled Endosseous Dental Implants in a Fluid Bath
ISO 14801 Fatigue Testing of Pre-Angled Endosseous Dental Implants in a Fluid Bath Braces Testing: Metal, Plastic, Ceramic Materials
Braces Testing: Metal, Plastic, Ceramic Materials









